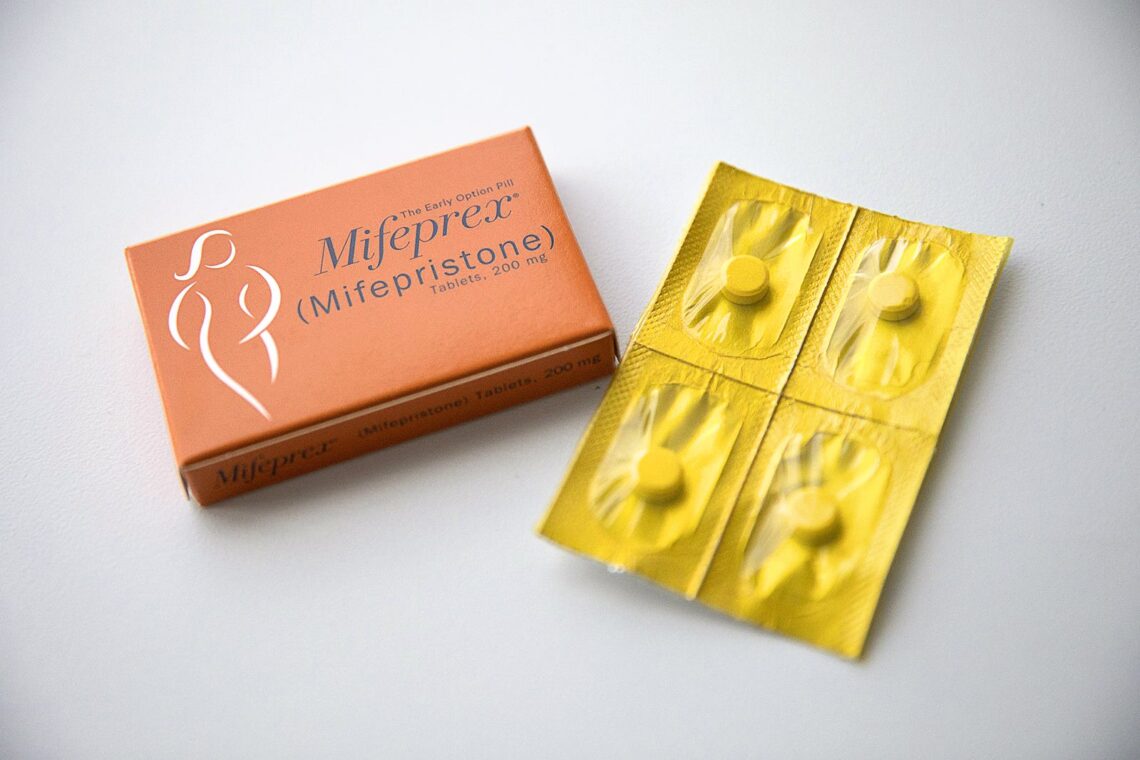
A medical journal has retracted two studies examining the safety of the abortion pill mifepristone after a federal judge in Texas cited them when ruling that the drug should be taken off the market.
The studies, both retracted because of methodology problems and conflicts of interest, claimed abortions involving mifepristone are associated with an increased risk of serious complications compared with procedural abortions. Those conclusions are in contrast with hundreds of studies in the past two decades that have found that mifepristone—currently approved by the U.S. Food and Drug Administration for use in abortion through 10 weeks of pregnancy—is safe and effective. Mifepristone is used in combination with the drug misoprostol in nearly all medication abortions in the U.S., and medication abortions constituted more than half of abortions nationwide in 2020.
A third study that was written by the same authors but not referenced by the judge was also retracted; it was about doctors who prescribe mifepristone. All three papers were published in Health Services Research and Managerial Epidemiology, which is published by Sage Journals. They appeared in the journal in 2019, 2021 and 2022.
On supporting science journalism
If you’re enjoying this article, consider supporting our award-winning journalism by subscribing. By purchasing a subscription you are helping to ensure the future of impactful stories about the discoveries and ideas shaping our world today.
The papers drew attention after the Alliance for Hippocratic Medicine, a group of antiabortion doctors and organizations, sued the U.S. Food and Drug Administration in November 2022. The alliance claimed that the FDA did not follow proper procedures in approving the drug more than two decades ago and that it has downplayed mifepristone’s risks. When filing its suit, the alliance also asked for a preliminary injunction to immediately remove mifepristone from the market.
In court documents, the U.S. Department of…
Read the full article here