First Weight-Loss Drug Gets Approval for Heart Disease. Here’s What We Know
The FDA recently approved semaglutide (Wegovy) for preventing serious heart conditions in some people, but questions remain about how it works
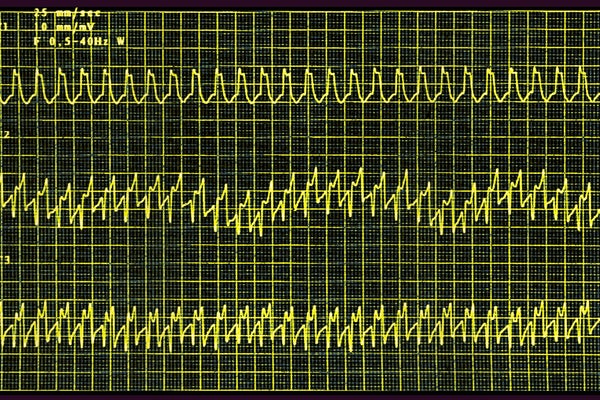
An electrocardiogram of a person with atrial tachycardia, a heart rhythm disorder in which people experience rapid palpitations.
James Cavallini/BSIP/Universal Images Group via Getty Images
The drug semaglutide, sold commercially as Ozempic or Wegovy, is well known for helping people lose significant amounts of weight quickly. Now the U.S. Food and Drug Administration has approved Wegovy, the version of semaglutide currently prescribed for weight loss, for preventing serious cardiovascular conditions in certain people who are at high risk.
The FDA’s March 8 announcement will allow doctors to prescribe Novo Nordisk’s Wegovy, which has a higher maximum dose of semaglutide than Ozempic does, to people who are overweight or obese and have had at least one cardiovascular event such as a heart attack or stroke. “It opens up a whole new group of patients for us,” says Nicholas Marston, a cardiologist at Brigham and Women’s Hospital.
Here’s what we know about semaglutide’s effects on cardiovascular disease and how it might work.
On supporting science journalism
If you’re enjoying this article, consider supporting our award-winning journalism by subscribing. By purchasing a subscription you are helping to ensure the future of impactful stories about the discoveries and ideas shaping our world today.
Who’s a good candidate for taking the drug to reduce cardiac risk?
The FDA’s approval expands the use of semaglutide to people with a BMI of 27 or higher (qualified as overweight or obese) with a history of cardiac events. But it’s unknown whether the drug would work as well at preventing cardiovascular disease in people with lower BMIs. And it still hasn’t been tested in people who may be at risk of cardiovascular disease…
Read the full article here